| 3-CHLOROPIVALOYL CHLORIDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 4300-97-4 |
|
| EINECS NO. | 224-311-8 | |
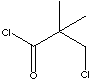
| FORMULA | ClCH2C(CH3)2COCl | |
| MOL WT. | 155.02 | |
| H.S. CODE | ||
| TOXICITY | ||
| SYNONYMS | 3-Chloro-2,2-Dimethylpropionyl Chloride | |
| Chloropivaloyl Chloride beta-Chloropivaloyl chloride Cloruro de 3-cloro-2,2-dimetilpropanoilo (Spanish) 3-Chlor-2,2-dimethylpropanoylchlorid (German) Chlorure de 3-chloro-2,2-dimé thylpropanoyle (French) | ||
| SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
clear to yellow liquid |
|
| MELTING POINT | ||
| BOILING POINT | ||
| SPECIFIC GRAVITY | 1.199 | |
| SOLUBILITY IN WATER | Reacts |
|
| AUTOIGNITION |
|
|
| pH | ||
| VAPOR DENSITY | 5.35 | |
| NFPA RATINGS | ||
| REFRACTIVE INDEX | 1.4530 | |
| FLASH POINT |
62 C |
|
| STABILITY | Stable under ordinary conditions. Moisture sensitive. | |
|
APPLICATIONS |
||
| Acid chlorides are used as very reactive intermediates to prepare carboxylic acid derivatives including anhydrides, esters and amides because of the two strong electron withdrawing chlorine and oxygen on the carbonyl compound, and positive charge carbon accordingly. It is easy for a weak nucleophile to attack the carbon. Acid chlorides are also reactive with Gilman reagents to prepare large molecules from small ones by replacing the halides with an organic group. 3-Chloropivaloyl chloride, , multi-methylated acetyl chloride with an additional chloride at the terminal carbon, is used as an intermediate for the manufacturing photographic developers, herbicides, pesticides and rubber additives. It is used as an initiator of chain reaction for vinyl chloride-propylene copolymers. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear to yellow liquid |
|
| CONTENT |
98.0% min |
|
INDIVIDUAL IMPURITY |
0.5% max | |
| TRANSPORTATION | ||
| PACKING | 240kgs in drum | |
| HAZARD CLASS | 8 (Packing Group: II) | |
| UN NO. | 1760 | |
| OTHER INFORMATION | ||
| Hazard Symbols: C, Risk Phrases: 34, Safety Phrases: 24/25 | ||
|
GENERAL DESCRIPTION OF PIVALIC ACID |
||
|
Pivalic acid is the shortest chain tert-carboxylic acid. Atoms within a molecule occupy space. When atoms are crowded together and overlapped electron clouds, van der Waals repulsions produce steric hindrance. Steric hindrance may influence conformational equilibria and reactivity. Although steric hindrance is sometimes a unfavorable structure due to less readily reaction, it can provide an escape from undesired side-reactions, can affect varying degrees of rate and energy and can produce the target derivatives which are more resistant to hydrolysis and oxidation than the derivatives from linear chain. Pivalic acid is a key intermediate for the target molecules which require hydrolytic stability and a variety of chemical resistance. Pivalic acid is used mainly in the form of chloride salt (pivaloyl chloride) which are obtained commercially from phosgene. It is used as an intermediate in the production of peroxides and peroxy-esters required for the polymer and agrochemical production. It is used as an intermediate to prepare pharmaceuticals ( Ampicillin, Amoxycillin, Cephalosporins). 2,2-Dimethylbutyric acid is the next shortest chain tert-carboxylic acid which have similar application with pivalic acid. |
||